
A higher bleeding tendency in 12-mo DAPT compared with 3-mo DAPT was more obvious in the pre-TIMI 2/3 group than in the pre-TIMI 0/1 group.Ĭlinical Trial Registration: URL. The occurrence of MACCE was similar between the two groups.
However, in the pre-TIMI 2/3 group, the occurrences of TIMI minor (adjusted hazard ratio : 0.294 p = 0.016) and major or minor bleeding (aHR: 0.483 p = 0.014) on intention-to-treat analysis were significantly higher in the 12-mo than in the 3-mo DAPT group. In the pre-TIMI 0/1 group, the primary and second outcomes were not significantly different between the 3-mo and 12-mo DAPT groups. The secondary outcomes were the occurrence of the individual components of TIMI bleedings and MACCE. The primary outcome was the occurrence of net adverse clinical events (NACE), defined as a composite of TIMI major bleeding and major adverse cardiac and cerebrovascular events (MACCE) within 12-mo following PCI. This was a post hoc analysis of the TICO trial. The incidence of TIMI-3 flow was 68% for patients receiving abciximab, 50 mg t-PA, and very-low-dose heparin.We investigated the impact of pre-percutaneous coronary intervention (pre-PCI) thrombolysis in myocardial infarction (TIMI) flow grade (pre-TIMI) on 3-month (3-mo) and 12-mo of dual antiplatelet therapy (DAPT) in patients with acute myocardial infarction (AMI). TIMI-3 flow rates at 90 minutes were 63% for the control patients who received t-PA alone (n=208), compared to 77% for patients receiving abciximab with 50mg t-PA and low-dose heparin (n=93). Simultaneously, patients were randomized to low-dose heparin or very low-dose heparin (30 U/kg bolus with 4U/kg/hr infusion). In the dose-confirmation phase, the investigators examined the abciximab with 50mg t-PA regimen. The major hemorrhage rate was 12% in the collective experience with streptokinase and abciximab. The rate of intracranial, retroperitoneal, or other major hemorrhage was 6% for the t-PA control, abciximab control, and pooled t-PA with abciximab arms.
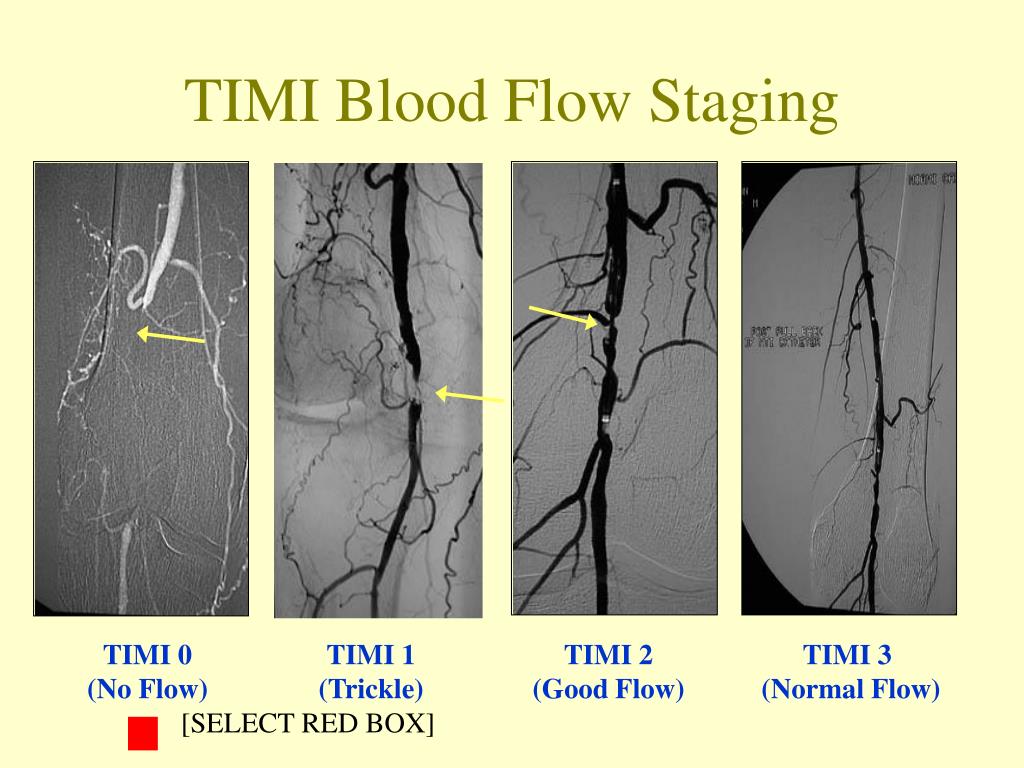
#TIMI 3 FLOW. PLUS#
The best t-PA arm (bolus plus 60-minute infusion) had 65% TIMI-3 flow at 60 minutes (n=51) and 72% TIMI-3 flow at 90 minutes (n=101). The control (t-PA alone) arms had TIMI-3 flow in 45% of patients at 60 minutes and 57% of patients at 90 minutes, consistent with previous angiographic studies of t-PA. The most promising regimen used 50mg of t-PA: a 15mg t-PA bolus, followed by 35mg administered over 60 minutes.Īngiographic measurements of the speed and extent of thrombolysis were evaluated in terms of 60 and 90 minute TIMI-3 flow. An arm combining abciximab with 1.5 million units of streptokinase arm was terminated early because of excess bleeding.Ībciximab therapy with t-PA produced TIMI-3 flow in 38-77% of patients. (For comparison, the TIMI-3 reperfusion rate was 30% for streptokinase alone in a meta-analysis of TIMI-1 and GUSTO-I results.)Ībciximab coupled with streptokinase produced TIMI-3 flow in 39-47% of patients. TIMI-3 flow at 90 minutes was achieved in 57% of patients given t-PA alone, compared to 32% of patients given abciximab alone. A blinded core laboratory interpreted 90-minute angiograms for 98% of patients. Four doses of streptokinase (500,000, 750,000, 1.25 million and 1.5 million units) were evaluated. Eight different t-PA regimens were tested, representing total doses of 20, 35, 50, and 65mg given as bolus, 30-, or 60- minute infusions. Patients who received abciximab (0.25 mg/kg bolus followed by 0.125 mcg/kg/min infusion for 12 hours) were further randomized to receive 1 of 12 thrombolytic doses, or no concomitant thrombolytic. Patients randomized to low-dose heparin with abciximab (either alone or with reduced-dose thrombolytic therapy) or standard-dose heparin (70 U/kg bolus followed by 15 U/kg/hr) with accelerated t-PA (up to 100mg).


 0 kommentar(er)
0 kommentar(er)
